Synthesis of nitrogen-containing fused-polycyclic compounds from tyramine derivatives using phenol dearomatization and cascade cyclization†
Abstract
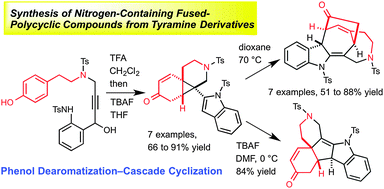
We developed a novel method of synthesizing nitrogen-containing fused-polycyclic compounds using tyramine derivatives as substrates. The method is based on the dearomatization of phenols via an intramolecular ipso-Friedel–Crafts allenylation and sequential bond-forming–cleavage reactions initiated by the construction of an indole skeleton. Structurally diverse fused-heterocycles were produced in reaction sequences requiring only a few steps.

 Please wait while we load your content...
Please wait while we load your content...