Synthesis and kinetic resolution of N-Boc-2-arylpiperidines†
Abstract
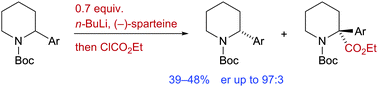
The chiral base n-BuLi/(−)-sparteine or n-BuLi/(+)-sparteine surrogate promotes kinetic resolution of N-Boc-2-arylpiperidines by asymmetric deprotonation. The enantioenriched starting material was recovered with yields 39–48% and ers up to 97 : 3. On lithiation then electrophilic quench, 2,2-disubstituted piperidines were obtained with excellent yields and enantioselectivities.
- This article is part of the themed collections: Celebrating our 2018 prize and award winners and In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...