Facile regiospecific synthesis of 2,3-disubstituted indoles from isatins†
Abstract
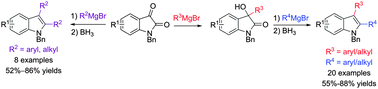
A facile regiospecific synthesis of 2,3-disubstituted indoles from isatins has been developed. Nucleophilic addition of Grignard reagents to commercially available isatins, followed by reduction with borane, afforded an array of structurally diverse 2,3-disubstituted indoles in moderate to good yields. The method described herein represents a novel and very simple approach to synthesize various 2,3-disubstituted indoles, extremely important structural motifs in the pharmaceutical industry and medicinal chemistry.

 Please wait while we load your content...
Please wait while we load your content...