Synthesis of nitrogen heterocycles via α-aminoalkyl radicals generated from α-silyl secondary amines under visible light irradiation†
Abstract
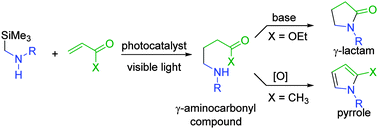
We have succeeded in the visible light-mediated synthetic use of α-aminoalkyl radicals derived from α-silyl secondary amines toward addition to α,β-unsaturated carbonyl compounds. The resulting γ-aminocarbonyl compounds are converted into γ-lactams and pyrroles in a one-pot process.

 Please wait while we load your content...
Please wait while we load your content...