Visible-light-induced direct C(sp3)–H difluromethylation of tetrahydroisoquinolines with the in situ generated difluoroenolates†
Abstract
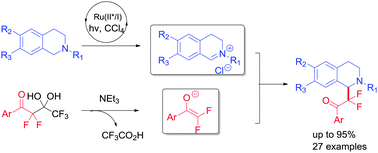
An effective approach to C1-difluoromethylated tetrahydroisoquinoline derivatives has been developed through C–H functionalization of tertiary amines by visible-light photoredox catalysis. This method uses stable, easily obtained α,α-difluorinated gem-diol as the CF2 source. The corresponding products were obtained in moderate to high yields at ambient temperature.

 Please wait while we load your content...
Please wait while we load your content...