Abstract
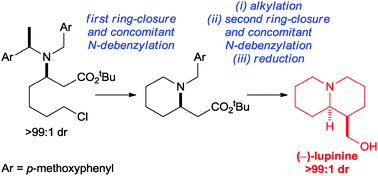
The asymmetric synthesis of (−)-lupinine was achieved in 8 steps, 15% overall yield and >99 : 1 dr from commercially available starting materials. The strategy used for the construction of the quinolizidine scaffold involved reaction of an enantiopure tertiary dibenzylamine via two sequential ring-closures which both occurred with concomitant N-debenzylation.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...