Cleavage of unactivated amide bonds by ammonium salt-accelerated hydrazinolysis†
Abstract
Hydrazinolysis of unactivated amide bonds is significantly accelerated by the addition of ammonium salts. The reactions proceed at 50–70 °C to give amines with broad substrate scope that outperforms existing amide bond cleavage reactions. Application to peptide and amino sugar derivatives is also demonstrated.
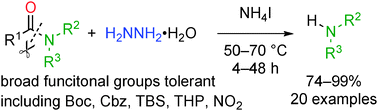

 Please wait while we load your content...
Please wait while we load your content...