A masked diboron in Cu-catalysed borylation reaction: highly regioselective formal hydroboration of alkynes for synthesis of branched alkenylborons†
Abstract
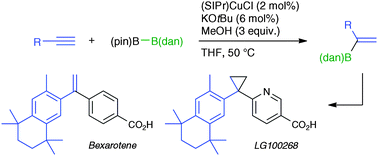
The use of a masked diboron as a boron source in the presence of a Cu–N-heterocyclic carbene (NHC) catalyst enables alkyl-, aryl-, heteroatom- and silyl-substituted terminal alkynes to undergo α-selective formal hydroboration to give diverse branched alkenylboron compounds exclusively. Synthetic potential of this α-selective hydroboration has been demonstrated by total synthesis of pharmaceutically significant bexarotene and LG100268.

 Please wait while we load your content...
Please wait while we load your content...