Copper-catalyzed tandem oxidative cyclization of cinnamamides with benzyl hydrocarbons through cross-dehydrogenative coupling†
Abstract
The copper-catalyzed tandem oxidative cyclization of cinnamamides with benzyl hydrocarbons through the direct cross-dehydrogenative coupling of sp3 and sp2 C–H bonds has been developed. Satisfactorily, ethers, alcohols and alkanes could also be used instead of benzyl hydrocarbons in this transformation, which allows rapid access to diverse dihydroquinolinones in one step.
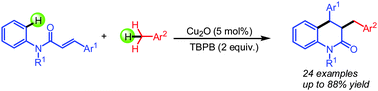

 Please wait while we load your content...
Please wait while we load your content...