Unusual formation of a N-heterocyclic germylene via homolytic cleavage of a C–C bond†
Abstract
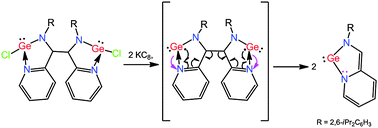
Reaction of the monoanionic radical salt IP˙−K+ (IP = (Py)CH(=NR); Py = C5H4N, R = 2,6-iPr2C6H3; α-iminopyridine) with GeCl2(dioxane) afforded compound (IPGeCl)2 (1) which produced red blocks of IPGe: (2), when treated with KC8 in toluene. 1 is a digermylene formed via C–C coupling between two carbon-centered radicals. 2 can be considered as an analogue of a N-heterocyclic carbene, which exhibits a five-membered GeC2N2 ring with one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. 2 is formed by two-electron reduction of 1 with cleavage of the two Ge–Cl bonds and the central C–C single bond.
C double bond. 2 is formed by two-electron reduction of 1 with cleavage of the two Ge–Cl bonds and the central C–C single bond.

 Please wait while we load your content...
Please wait while we load your content...