Binaphthol-derived phosphoric acids as efficient chiral organocatalysts for the enantiomer-selective polymerization of rac-lactide†
Abstract
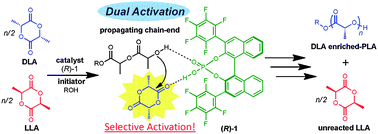
A high enantiomer-selectivity for the polymerization of rac-lactide was achieved using chiral binaphthol-derived monophosphoric acids as organocatalysts. During the polymerization, D-lactide (DLA) preferentially polymerized via kinetic resolution with the maximum selectivity factor (kD/kL) of 28.3. The selective polymerization of DLA was derived from a dual activation, i.e., monomer activation and chain-end activation.

 Please wait while we load your content...
Please wait while we load your content...