Rational design of sulfoxide–phosphine ligands for Pd-catalyzed enantioselective allylic alkylation reactions†
Abstract
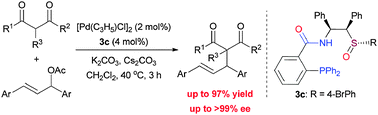
A new type of chiral sulfoxide–phosphine ligands have been developed by a rational combination of two privileged scaffolds for Pd-catalyzed asymmetric allylic alkylation reactions. Under optimized conditions, generally high yields (up to 97%) and excellent enantioselectivities (up to >99% ee) were obtained.

 Please wait while we load your content...
Please wait while we load your content...