Discovering complexes containing a metal–metal quintuple bond: from theory to practice
Abstract
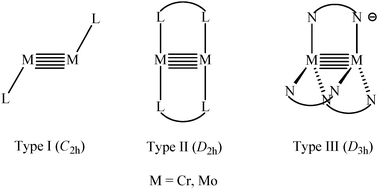
Although the field of metal-to-metal multiple bonding has been considered as mature, it was recently reinvigorated by the discovery of quintuple bonded dinuclear complexes. Initiated by theoretical studies, quintuple bonding was promoted by the recognition of the first isolable quintuple bonded chromium phenyl dimer Ar′CrCrAr′ (Ar′ = 2,6-(2,6-iPr2C6H3)2C6H3) by Power and co-workers in 2005. Soon afterwards, many dinuclear group VI metal-to-metal quintuple bonded complexes stabilized by sterically hindered N-based bidentate ligands were subsequently characterized. All these remarkable compounds feature two or three bridging ligands, so each metal is two to three coordinate with respect to the ligands. Of particular interest is that they all have extremely short metal–metal bond lengths. These low-coordinate homo-univalent quintuple bonded dinuclear species not only display remarkable bonding schemes between two metal atoms, but also show interesting reaction chemistry with unsaturated hydrocarbons and small inorganic molecules. Herein, we review recent advances in quintuple bond chemistry including the synthesis, characterization and reactivity of quintuple bonded complexes.

 Please wait while we load your content...
Please wait while we load your content...