New catalytic model systems of tyrosinase: fine tuning of the reactivity with pyrazole-based N-donor ligands†
Abstract
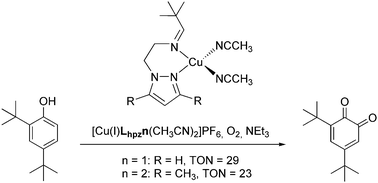
Two new Cu(I) complexes have been synthesized and investigated as model systems of the enzyme tyrosinase. The corresponding ligands are based on a combination of an imine function with two different pyrazole groups. The reactivity of the prepared systems with respect to the conversion of monophenols to the corresponding ortho-quinones is investigated. The resulting data are compared to results obtained for other catalytic model systems of tyrosinase.
- This article is part of the themed collection: Biological oxidation reactions: mechanisms and design of new catalysts

 Please wait while we load your content...
Please wait while we load your content...