Enantioselective synthesis of diaryl aziridines using tetrahydrothiophene-based chiral sulfides as organocatalysts†
Abstract
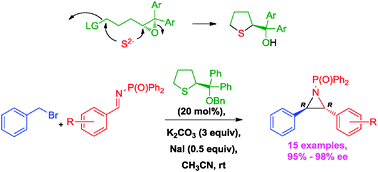
This work describes catalytic and asymmetric aziridinations (15 examples, 95–98% ee) of benzyl bromide and imines via the imino Corey–Chaykovsky reaction using (thiolan-2-yl)diarylmethanol benzyl ether as an organocatalyst. The catalyst and analogues thereof were prepared through an expeditious and efficient synthetic route featuring a double nucleophilic substitution and Shi epoxidation as key steps.

 Please wait while we load your content...
Please wait while we load your content...