An antibody–magnetic nanoparticle conjugate-based selective filtration method for the rapid colorimetric detection of Listeria monocytogenes
Abstract
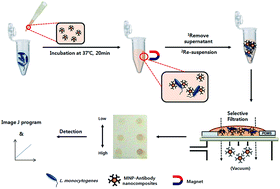
In the present study, a method for the rapid colorimetric detection of Listeria monocytogenes based on selective filtration was developed by using monoclonal antibody (MAb)–magnetic nanoparticle (MNP) conjugates with a nitrocellulose filter membrane possessing a pore size of 1.2 μm. The conjugates bound and unbound to L. monocytogenes after being isolated on a magnet were directly passed through a nitrocellulose filter membrane by vacuum pressure, and the color signals from conjugate-bacteria that remain on the membrane are measured, reflecting the amount of bacteria in a sample. The capture efficiencies of the conjugates were confirmed to be 48% to 89% for 103, 102, and 101 cells per mL of L. monocytogenes. After optimization, 1.2 × 101 cells of L. monocytogenes were detectable in a pure pathogen solution from the standard curve, and the cut-off value for artificially inoculated vegetables by the method was 1 × 102 cells per g. Although weak cross-reactivity to Listeria spp. and Staphylococcus aureus was observed, the method was confirmed to be highly specific to L. monocytogenes. The method can be concluded within 35 min, yielding results in a shorter or similar period to recently reported antibody-immobilized MNP-based methods without additional steps such as dissociation of the target bacteria from MNP–MAb or enrichment. Therefore, the method could be used as a rapid, sensitive, and cost-effective tool for the detection of L. monocytogenes in various vegetables at a minimum detection level of 1 × 102 cells per g.

 Please wait while we load your content...
Please wait while we load your content...