Single-step and reagentless analysis of genetically modified soybean DNA with an electrochemical DNA biosensor
Abstract
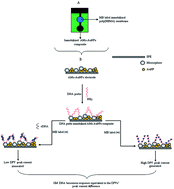
In many electrochemical DNA based biosensors, a redox indicator is required to be introduced separately to indicate the DNA hybridization event. In this work, we have developed a simple procedure for voltammetric determination of CaMV 35S gene modified DNA without the need for introduction of a redox indicator. The DNA biosensor contains an immobilized DNA probe and also a methylene blue redox indicator that is able to slow release during the hybridization event. The biosensor was constructed from a screen printed carbon paste electrode (SPE) coated with an acrylic microsphere (AM)–gold nanoparticle (AuNP) composite immobilized with single-stranded DNA probes, whilst methylene blue (MB) was immobilized in the hydrogel poly(2-hydroxyethyl methacrylate) membrane located next to the electrode. Genetically modified (GM) DNA was examined based on the reduction in the MB cathodic peak current (ipc) signal, which was ascribed to the DNA hybridization event, via the differential pulse voltammetry (DPV) method. The ipc current signal of MB after DNA hybridization with target CaMV 35S gene DNA was linearly related to the logarithmic target DNA concentration ranging from 2.0 × 10−12 to 2.0 × 10−7 M (R2 = 0.989) with a limit of detection (LOD) at 1.40 × 10−13 M. The proposed AM–AuNP composite DNA electrode gave satisfactory reproducibility performance with <10% (n = 5) relative standard deviation (RSD). The recoveries between 94.1 ± 2.2% and 103.7 ± 8.2% (n = 5) were obtained when the DNA biosensor was used for GM DNA determination in GM soybean DNA samples. The DNA biosensor based on the AM–AuNP composite deposited SPE and immobilized MB exhibited higher sensitivity by single-step analysis compared with conventional electrochemical sensors.

 Please wait while we load your content...
Please wait while we load your content...