A solid phase extraction method for analysis of the iron–humic acid complex in natural water
Abstract
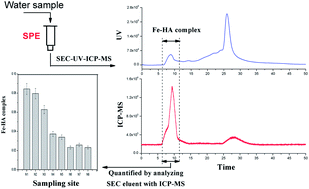
Extracting the iron–humic acid (Fe–HA) complex from natural waters is difficult, since there is a lack of standards and many impurities are co-extracted with the target analytes. In this study, a laboratory synthesized Fe–HA complex was used as a standard to develop a simple solid phase extraction (SPE) method for analysis of the Fe–HA complex in natural water. The Fe–HA complex in the SPE extract was separated from the matrix and analyzed by size exclusion chromatography (SEC) combined with ultraviolet spectrophotometry. The Fe–HA complex was quantified as the molar concentration of HA bound Fe, which was determined by analyzing the SEC eluent with inductively coupled plasma mass spectrometry. The results suggested that optimized extraction could be achieved with an ENVI-18 cartridge as the sorbent, methanol as the eluent, and a flow rate of sample loading of 10 ± 2 mL min−1, and without adjusting the sample pH. Under the proposed SPE conditions, the linear range of the Fe–HA complex was 0.052 to 0.301 μmol L−1. The detection limit (S/N = 3.0) was found to be 0.0012 μmol L−1. The recovery of the spiked Fe–HA complex from a natural river water sample was 76.8% and an acceptable repeatability with a relative standard deviation of 5.2% was achieved. Moreover, the developed SPE method was successfully applied to analyze the Fe–HA complex in the Jiulongjiang River. It was found that the concentrations of the complex were in the range 0.023–0.085 μmol L−1, showing a gradual decrease from upstream to downstream.
- This article is part of the themed collection: Emerging analytical methods for global energy and climate issues

 Please wait while we load your content...
Please wait while we load your content...