Enantiomeric separation of 2-arylpropionic acid nonsteroidal anti-inflammatory drugs and β-blockers by RP-HPLC using an amylose chiral stationary phase for the enantioselective skin permeation study
Abstract
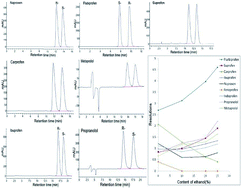
The enantioselectivities of eight 2-arylpropionic acid nonsteroidal anti-inflammatory drugs (2-APA NSAIDs) and two β-blockers on an amylose-tris(3,5-dimethylphenylcarbamate)-based Chiralpak AD-RH (150 × 4.6 mm, 5 μm) column were studied in reverse phase (RP) elution mode. The influencing factors such as mobile phase composition, organic modifiers, and column temperature were investigated. For most of the selected NSAIDs, baseline enantioseparation was achieved using a mobile phase composition of acetonitrile–0.05% trifluoroacetic acid aqueous solution. For the β-blockers, baseline enantioseparation was achieved using a mobile phase composition of acetonitrile–borate buffer solution with 0.1% triethylamine (pH 9.0; 10 mM). Our study has demonstrated for the first time that the addition of an organic modifier, ethanol, in the mobile phase dramatically improves the resolution of the analytes such as flurbiprofen, suprofen, propranolol and metoprolol under reverse phase chromatographic conditions. The methods for the enantioselective determination of ibuprofen, flurbiprofen and propranolol were validated. The enantioselective skin permeation studies of propranolol hydrochloride presented here showed that there was no observable enantioselective permeation of propranolol across the excised rat skin, but L-linalool, a chiral enhancer, resulted in enhanced enantioselectivity, with a better permeation enhancing effect on R-propranolol than on S-propranolol and RS-propranolol across the excised rat skin.

 Please wait while we load your content...
Please wait while we load your content...