Microarray evaluation of the effects of lectin and glycoprotein orientation and data filtering on glycoform discrimination†
Abstract
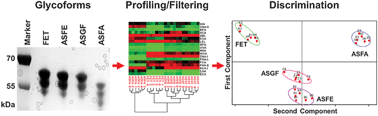
Affinity molecules offer promise in the development of inexpensive, high-throughput methods that are complementary to traditional carbohydrate analysis techniques such as chromatography and mass spectrometry. Lectins are carbohydrate binding proteins which have been effectively used in many applications, however, their broad and label-dependent specificities can make data interpretation with known structures difficult and their unambiguous use for analysis of unknowns impossible. To evaluate the usefulness of lectins in distinguishing closely related structural glycoforms of glycoproteins, the well-characterised glycoprotein bovine fetuin (Fet), along with three additional Fet glycoform populations produced by enzymatic and chemical means, were profiled using a microarray consisting of 43 lectins with affinities covering a variety of carbohydrate structures. Fully-sialylated and fully-desialylated forms of Fet, as well as two intermediate forms with partial sialylation and galactosylation, generated distinct profiles. Using stringent data filtering, the total number of printed lectins required to distinguish four heterogeneous Fet glycoform pools was reduced to just eight and also enabled stronger correlation between known Fet glycan structures and reported lectin specificities. A major application of a high-throughput lectin profiling approach would be monitoring glycosylation on biopharmaceutical proteins, but a potential complication may be the presence of interfering molecules in the solvent matrix. This possibility was evaluated with an expanded dataset including an additional five partially-inhibitory conditions and the samples could also be individually discriminated with seven lectins using this strategy. Based on known carbohydrate structures, several lectins gave unexpected responses in the lectin microarray format. Two of the Fet glycoforms were also printed in a microarray format and profiled by a panel of thirteen fluorescently-labeled lectins to evaluate the performance and specificity of binding in another orientation. Seven lectins differed in behaviour between platforms which demonstrated that lectin performance is also format-dependent. Together, these findings demonstrate the utility of lectin microarray profiling for selective identification of glycoprotein glycoforms even with interfering molecules present. Also highlighted is the use of stringent data filtering to more accurately correlate profile data to glycan structure, as well as the importance of evaluating lectin performance and structural correlation in the intended platform.

 Please wait while we load your content...
Please wait while we load your content...