Electrolytic valving isolation of cell co-culture microenvironment with controlled cell pairing ratios†
Abstract
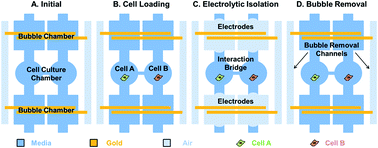
Cancer–stromal interaction is a critical process in tumorigenesis. Conventional dish-based co-culture assays simply mix two cell types in the same dish; thus, they are deficient in controlling cell locations and precisely tracking single cell behavior from heterogeneous cell populations. Microfluidic technology can provide a good spatial–temporal control of microenvironments, but the control has been typically realized by using external pumps, making long-term cultures cumbersome and bulky. In this work, we have presented a cell–cell interaction microfluidic platform that can accurately control the co-culture microenvironment by using a novel electrolytic cell isolation scheme without using any valves or pneumatic pumps. The proposed microfluidic platform can also precisely control the number of interacting cells and pairing ratios to emulate cancer niches. More than 80% of the chambers captured the desired number of cells. The duration of cell isolation can be adjusted by electrolytic bubble generation and removal. We have verified that the electrolytic process has a negligible effect on cell viability and proliferation in our platform. To the best of our knowledge, this work is the first attempt to incorporate electrolytic bubble generation as a cell isolation method in microfluidics. For proof of feasibility, we have performed cell–cell interaction assays between prostate cancer (PC3) cells and myoblast (C2C12) cells. The preliminary results demonstrated the potential of using electrolysis for micro-environmental control during cell culture. Also, the ratio controlled cell–cell interaction assays were successfully performed which showed that the cell pairing ratios of PC3 to C2C12 affected the proliferation rate of myoblast cells due to increased secretion of growth factors from prostate cancer cells.

 Please wait while we load your content...
Please wait while we load your content...