Tailoring recognition clefts from non-specific recognition matrices in mixed molecular arrays†
Abstract
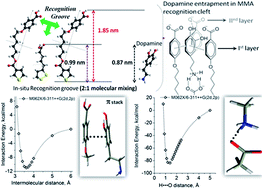
Multi-component organic interfaces with molecular-level mixing were prepared by integrating benzoic acid appended thiophene amphiphile [4-(6-(thiophene-3-carbonyloxy)hexyloxy)benzoic acid] (T6BA) and (±)-α-lipoic acid onto the Au surface. On a flat surface with infinite radii of curvature, T6BA and (±)-α-lipoic acid, endowed with chemically distinct end-groups, provided sufficient length mismatch to gain conformational entropy leading to stripe-like patterns when the immiscible ligands co-adsorbed. Good quality multi-component organic interfaces and molecular islands could be fabricated via composition variation of the participating ligands. Host–guest chemistry between benzoic acids and β-cyclodextrin was used to confirm the molecular-level mixing. T6BA and (±)-α-lipoic acid, each being a non-specific recognition matrix for dopamine, could thus be organized into mixed molecular arrays having well defined cavities for guest inclusion. This mixed molecular array behaved as a ‘recognition matrix’ for dopamine (DA, 15 nm) in the presence of ascorbic acid (AA). The surface patterns described here on a flat surface should in principle be applicable to other geometrical structures like spheres and cylinders. Further, charge transfer through the T6BA self-assembled monolayers depended on the anion type present in the supporting electrolyte, monitored through cyclic voltammetry.

 Please wait while we load your content...
Please wait while we load your content...