Post-synthetic modifications of as-made zeolite frameworks near the structure-directing agents†
Abstract
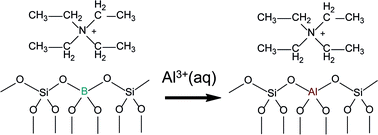
As-made zeolite B-Beta was converted to the Al form with aqueous 1 M Al(NO3)3 solutions at 448, 423 and 393 K under hydrothermal conditions. Almost complete removal of framework boron was observed at 448 K in seven days while tetraethylammonium cations (TEA+) are in the pore system. At the same time aluminium was inserted in the zeolite. 1H–11B and 1H–27Al REAPDOR NMR data show that TEA+–BO4/2− ion pair interactions are quantitatively replaced by TEA+–AlO4/2− interactions. This exchange reaction is a thermally activated process which consists of three contributions. The initial reaction in the first step removes defect sites near the crystal surface by aluminium insertion within the first day at 448 K. Subsequently, boron is replaced by aluminium in the bulk of the crystals in the second step. The estimated activation energy of this second process is Ea = 80 ± 15 kJ mol−1. This reaction step is consistent with a solid state migration of framework atoms under hydrolysis and condensation of bonds and/or a coupled dissolution–reprecipitation mechanism. In the last step, a faster exchange reaction takes place due to the formation of mesopores in the crystals. The reverse reaction on Al-Beta does not exchange boron for aluminium, using boric acid solutions, but boron is inserted into the framework while defect sites are removed. As-made B-ZSM-12 shows similar B/Al exchange as B-Beta. No reaction was observed for zeolite B-ZSM-5 with tetrapropyl-ammonium cations in the pores.

 Please wait while we load your content...
Please wait while we load your content...