Nitrogen-self-doped graphene-based non-precious metal catalyst with superior performance to Pt/C catalyst toward oxygen reduction reaction†
Abstract
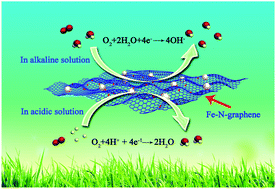
A new, simple and scalable synthesis methodology is invented for an N-self-doped graphene-based non-precious Fe catalyst (Fe–N-graphene) for the oxygen reduction reaction (ORR) both in acidic and alkaline media. The electrochemical characterization shows that this Fe–N-graphene catalyst possesses outstanding electrocatalytic ORR activity (similar to Pt/C catalyst in alkaline media and slightly lower in acidic media), and both superior stability and fuel (methanol and CO) tolerance to Pt/C catalysts. We believe that this is the first time for a non-precious metal catalyst to have superior ORR performance to Pt/C catalyst. In addition, our synthesis methodology can be scaled up for the mass production of N-self-doped graphene-based fuel cell non-noble metal catalysts and other nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...