Efficient hydrogen/oxygen evolution and photocatalytic dye degradation and reduction of aqueous Cr(vi) by surfactant free hydrophilic Cu2ZnSnS4 nanoparticles†
Abstract
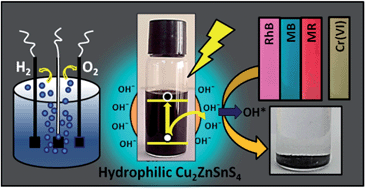
A single type of semiconductor material, surfactant free hydrophilic Cu2ZnSnS4 (CZTS) nanoparticles, for the first time has been explored as a three way heterogeneous catalyst in the electrocatalytic hydrogen evolution reaction (HER), photocatalytic degradation of polluting dyes and photocatalytic reduction of carcinogenic and mutagenic Cr(VI) to nontoxic Cr(III). Ultrafine surfactant free kesterite nanoparticles, CZTS, (∼4 nm) having an optimum band gap of 1.75 eV and electrical resistivity of 0.6 Ω m were used for various applications. The as-synthesized CZTS NPs showed promising electrocatalytic activity for the HER as well as the oxygen evolution reaction (OER) with a lower Tafel slope and excellent recyclable catalytic efficiency. Rhodamine B, methyl red and methylene blue dyes were degraded rapidly and efficiently in a photocatalytic pathway by the as-synthesized CZTS nanoparticles with only 5% activity loss in degradation after the 10th cycle. Similarly, a higher rate constant and activity parameter were observed for the photocatalytic reduction of hexavalent chromium ions. The high catalytic performance of the present CZTS NPs over other catalysts under indoor light conditions in the absence of any expensive noble metals and harsh reducing/oxidizing agents have been attributed to the size, surface charge and electronic effect of the nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...