Nanoconfinement of Mg6Pd particles in porous carbon: size effects on structural and hydrogenation properties†
Abstract
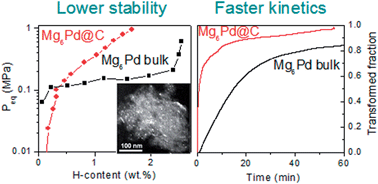
Mg6Pd nanoparticles as small as 4 nm have been synthesized inside the pores of porous carbon. They are formed by infiltration of Mg on previously formed Pd nanoparticles dispersed into carbon. Their crystalline structure, as evaluated by X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS), differs from bulk Mg6Pd since their particle size is close to the large crystal cell (∼2 nm) of this intermetallic compound. Indeed, as compared to bulk Mg6Pd, the nanoparticles exhibit a simpler crystallographic arrangement and a higher atomic disorder. Both thermodynamic and kinetic H-sorption properties of Mg6Pd nanoparticles differ from those of bulk Mg6Pd. The H-kinetics of the Mg6Pd nanoparticles are significantly faster than bulk and are stable for at least 10 sorption cycles. Thermodynamic destabilization of the hydrided state is also observed for Mg6Pd nanoparticles. Changes in the hydrogenation properties are attributed to nanosizing as well as to the modified structure of the nanoparticles as compared to bulk Mg6Pd.

 Please wait while we load your content...
Please wait while we load your content...