Electrocatalysis of the hydrogen-evolution reaction by electrodeposited amorphous cobalt selenide films†
Abstract
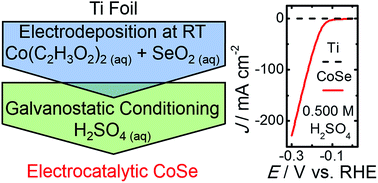
Using an electrochemical method under ambient conditions, crystallographically amorphous films of cobalt selenide have been deposited from aqueous solution onto planar Ti supports. These films have been evaluated as electrocatalysts for the hydrogen-evolution reaction. In 0.500 M H2SO4, the cobalt selenide films required an overpotential of ∼135 mV to drive the hydrogen-evolution reaction at a benchmark current density of −10 mA cm−2. Galvanostatic measurements indicated stability of the electrocatalytic films for >16 h of continuous operation at −10 mA cm−2. The facile preparation method, and the activity of the cobalt selenide films, suggest that electrodeposited metal chalcogenides are potentially attractive earth-abundant electrocatalysts for the hydrogen-evolution reaction.

 Please wait while we load your content...
Please wait while we load your content...