Molybdenum carbide–carbon nanocomposites synthesized from a reactive template for electrochemical hydrogen evolution
Abstract
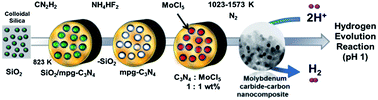
Molybdenum carbide nanocrystals (Mo2C) with sizes ranging from 3 to 20 nm were synthesized within a carbon matrix starting from a mesoporous graphitic carbon nitride (mpg-C3N4) template with confined pores. A molybdenum carbide phase (Mo2C) with a hexagonal structure was formed using a novel synthetic method involving the reaction of a molybdenum precursor with the carbon residue originating from C3N4 under nitrogen at various temperatures. The synthesized nanocomposites were characterized using powder X-ray diffraction (XRD), temperature-programmed reaction with mass spectroscopy (MS), CHN elemental analyses, thermogravimetric analyses (TGA), nitrogen sorption, X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). The results indicated that the synthesized samples have different surface structures and compositions, which are accordingly expected to exhibit different electrocatalytic activities toward the hydrogen evolution reaction (HER). Electrochemical measurements demonstrated that the sample synthesized at 1323 K exhibited the highest and most stable HER current in acidic media, with an onset potential of −100 mV vs. RHE, among the samples prepared in this study. This result is attributed to the sufficiently small particle size (∼8 nm on average) and accordingly high surface area (308 m2 g−1), with less oxidized surface entrapped within the graphitized carbon matrix.

 Please wait while we load your content...
Please wait while we load your content...