Possible correlation between enthalpies of formation and redox potentials in LiMSO4OH (M = Co, Fe, Mn), Li-ion polyanionic battery cathode materials
Abstract
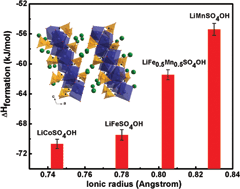
The thermodynamic stabilities of lithium hydroxysulfates of general formula LiMSO4OH (M = Co, Fe, Mn) with layered and tavorite structures have been investigated using isothermal acid solution calorimetry. These compounds have been explored as sustainable F-free alternatives to F-based flurosulfate cathode materials. The energetic trends for layered LiMSO4OH (M = Co, Fe and Mn) samples generally showed a decrease in stability with an increase in ionic radius (Co2+ to Mn2+), reflecting weaker M–O bonds and increasing structural distortions. The low symmetry tavorite LiFeSO4OH with a structure containing corner-shared octahedral chains is less stable than layered LiFeSO4OH with a more symmetric edge-shared octahedral structure. Structural distortions within the metal octahedra as well as changes in sulfate bonding and symmetry of the SO42− groups appear to control the thermodynamic and electrochemical behavior of LiMSO4OH (M = Co, Fe and Mn) materials. Both redox potential and thermodynamic stability of layered LiMSO4OH (M = Co, Fe and Mn) can be correlated to the lowering of the sulfate bonding symmetry in the structure from C3v to C2v.

 Please wait while we load your content...
Please wait while we load your content...