Asparagine-selective cleavage of peptide bonds through hypervalent iodine-mediated Hofmann rearrangement in neutral aqueous solution†
Abstract
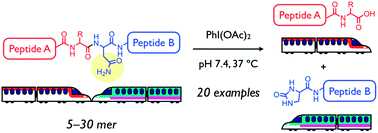
Amide bonds of peptides and proteins are generally unreactive toward hydrolysis, but backbone amide bond cleavage at a specific amino acid-site in an aqueous neutral solution at mild temperature could have many applications. Chemical cleavage methods that complement enzymatic digestion should facilitate the determination of primary structures for peptides and proteins, especially for substrates containing unnatural amino acids and/or chemical modifications that are resistant to enzymatic hydrolysis. As a new entry of site-selective chemical peptide bond cleavage, an asparagine-selective method using diacetoxyiodobenzene (DIB) is described herein. DIB-mediated Hofmann rearrangement at the primary amide moiety of an Asn side chain afforded a five-membered N-acylurea intermediate that was successively hydrolyzed into two peptide fragments. The Asn-selective peptide bond cleavage proceeded in aqueous neutral solution at 37 °C for various oligopeptides (20 examples) with a protected N-terminal, including a disulfide bond-containing peptide, biologically active peptides, and [Pyr11]Aβ11–40, which is associated with Alzheimer’s disease. An unnatural peptide sequence comprising D-amino acids was also successfully cleaved as well. Moreover, this method was used to determine oxidation sites of a photo-oxidized Aβ3–16 derivative that was resistant to enzymatic cleavage.

 Please wait while we load your content...
Please wait while we load your content...