Ir-catalyzed intermolecular asymmetric allylic dearomatization reaction of indoles†
Abstract
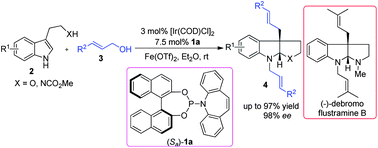
An Ir-catalyzed intermolecular asymmetric dearomatization reaction of substituted indoles with allylic alcohols has been realized to afford the corresponding indoline derivatives in 71–97% yield with up to 98% ee in the presence of the Lewis acid Fe(OTf)2. This methodology features the enantioselective construction of all-carbon quaternary stereogenic centers of prochiral nucleophiles and its utility has been demonstrated in the asymmetric total synthesis of (−)-debromoflustramine B.

 Please wait while we load your content...
Please wait while we load your content...