Organocatalytic nitrenoid transfer: metal-free selective intermolecular C(sp3)–H amination catalyzed by an iminium salt†
Abstract
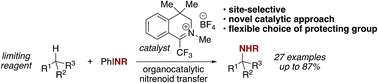
This report details the first organocatalytic method for nitrenoid transfer and its application to intermolecular, site-selective C(sp3)–H amination. The method utilizes a trifluoromethyl iminium salt as the catalyst, iminoiodinanes as the nitrogen source, and substrate as the limiting reagent. Activated, benzylic, and aliphatic substrates can all be selectively functionalized in yields up to 87%. A mechanistic proposal for the observed reactivity supported by experimental evidence invokes the intermediacy of a diaziridinium salt or related organic nitrenoid, species that have not been previously explored for the purpose of C–H amination. Finally, examples of late-stage functionalization of complex molecules highlight the selectivity and potential utility of this catalytic method in synthesis.


 Please wait while we load your content...
Please wait while we load your content...