Water splitting with polyoxometalate-treated photoanodes: enhancing performance through sensitizer design†
Abstract
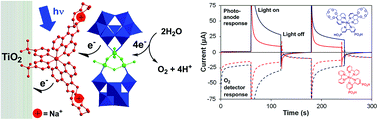
Visible light driven water oxidation has been demonstrated at near-neutral pH using photoanodes based on nanoporous films of TiO2, polyoxometalate (POM) water oxidation catalyst [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10− (1), and both known photosensitizer [Ru(bpy)2(H4dpbpy)]2+ (P2) and the novel crown ether functionalized dye [Ru(5-crownphen)2(H2dpbpy)](H22). Both triads, containing catalyst 1, and catalyst-free dyads, produce O2 with high faradaic efficiencies (80 to 94%), but presence of catalyst enhances quantum yield by up to 190% (maximum 0.39%). New sensitizer H22 absorbs light more strongly than P2, and increases O2 quantum yields by up to 270%. TiO2-2 based photoelectrodes are also more stable to desorption of active species than TiO2–P2: losses of catalyst 1 are halved when pH > TiO2 point-of-zero charge (pzc), and losses of sensitizer reduced below the pzc (no catalyst is lost when pH < pzc). For the triads, quantum yields of O2 are higher at pH 5.8 than at pH 7.2, opposing the trend observed for 1 under homogeneous conditions. This is ascribed to lower stability of the dye oxidized states at higher pH, and less efficient electron transfer to TiO2, and is also consistent with the 4th1-to-dye electron transfer limiting performance rather than catalyst TOFmax. Transient absorption reveals that TiO2–2–1 has similar 1st electron transfer dynamics to TiO2–P2–1, with rapid (ps timescale) formation of long-lived TiO2(e−)–2–1(h+) charge separated states, and demonstrates that metallation of the crown ether groups (Na+/Mg2+) has little or no effect on electron transfer from 1 to 2. The most widely relevant findings of this study are therefore: (i) increased dye extinction coefficients and binding stability significantly improve performance in dye-sensitized water splitting systems; (ii) binding of POMs to electrode surfaces can be stabilized through use of recognition groups; (iii) the optimal homogeneous and TiO2-bound operating pHs of a catalyst may not be the same; and (iv) dye-sensitized TiO2 can oxidize water without a catalyst.
- This article is part of the themed collection: Global Energy Challenges: Solar Energy

 Please wait while we load your content...
Please wait while we load your content...