A highly enantioselective acyl-Mannich reaction of isoquinolines with aldehydes promoted by proline derivatives: an approach to 13-alkyl-tetrahydroprotoberberine alkaloids†
Abstract
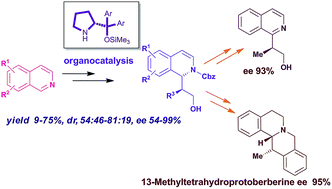
The highly stereoselective addition of aldehydes to isoquinolines, promoted by the Hayashi–Jørgensen secondary amine catalyst, is described. The procedure has a wide scope, with CbzCl or Boc2O used to activate isoquinoline to nucleophilic addition, allowing for the facile generation of useful synthetic intermediates in high enantiomeric excesses. The products obtained are synthetic intermediates for the synthesis of tetrahydroprotoberberine alkaloids. This methodology has been applied in the first enantioselective synthesis of 13-methyl tetrahydroprotoberberine, as reported herein.

 Please wait while we load your content...
Please wait while we load your content...