Complete stereodivergence in the synthesis of 2-amino-1,3-diols from allenes†
Abstract
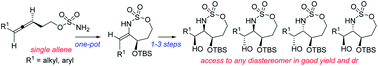
Amine-containing stereotriads (‘triads’), where the nitrogen is embedded in an array of three contiguous, heteroatom-bearing chiral carbons, are key motifs in numerous bioactive natural products. Allene aziridination provides convenient access to amine triads where the position of the nitrogen and the identities of the accompanying heteroatoms can be readily manipulated. However, stereochemical flexibility, where a single allene can be selectively transformed into any possible diastereomer of a specific triad, has been elusive. Herein, we describe studies to understand how both reagent and substrate control can be effectively employed in the stereodivergent oxidative amination of allenes, with transfer of the axial chirality of an enantioenriched precursor to point chirality in each possible diastereomeric 2-amino-1,3-diol product. Application of this flexible strategy to the synthesis of all four stereoisomers of the natural product detoxinine is also presented.

 Please wait while we load your content...
Please wait while we load your content...