Improved overall water splitting with barium tantalate mixed oxide composites
Abstract
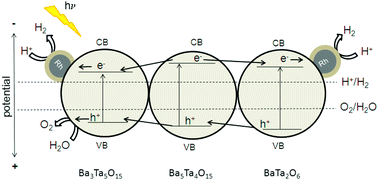
The combination of effective charge carrier separation and improved electron transfer in highly crystalline barium tantalate composites modified with Rh–Cr2O3 core–shell co-catalyst systems induces enhanced activity for overall water splitting (OWS) with stoichiometric amounts of H2 and O2 (2 : 1). A sol–gel route employing complexing reagents was investigated to prepare selectively defined mixed oxide materials with improved surface areas and smaller particle sizes compared to the conventional solid state reaction (SSR). The catalytic activities of the materials are investigated in photocatalytic test reactions for hydrogen production and overall water splitting. The formation of Rh–Cr2O3 core–shell co-catalyst systems for water splitting is evidenced by transmission electron microscopy (TEM) and X-ray Photoelectron Spectroscopy (XPS). Moreover, we developed new and highly active barium tantalate composites for hydrogen generation from aqueous methanol solutions under UV-light, which show the highest hydrogen evolution rate for a three-component composite consisting of Ba5Ta4O15/Ba3Ta5O15/BaTa2O6. Hydrogen rates of more than 6 mmol h−1 can be achieved without any co-catalyst. Using Rh–Cr2O3 core–shell co-catalysts on these three-component composites simultaneous generation of H2 and O2 from pure water splitting reaches rates up to 70% higher than for the pure Ba5Ta4O15.

 Please wait while we load your content...
Please wait while we load your content...