Preparation and properties of an MnIV–hydroxide complex: proton and electron transfer at a mononuclear manganese site and its relationship to the oxygen evolving complex within photosystem II†
Abstract
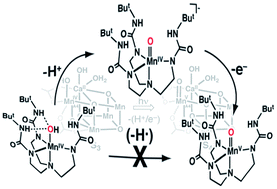
Photosynthetic water oxidation is catalyzed by a Mn4O5Ca cluster with an unprecedented arrangement of metal ions in which a single manganese center is bonded to a distorted Mn3O4Ca cubane-like structure. Several mechanistic proposals describe the unique manganese center as a site for water binding and subsequent formation of a high valent Mn–oxo center that reacts with a M–OH unit (M = Mn or CaII) to form the O–O bond. The conversion of low valent Mn–OHn (n = 1, 2) to a Mn–oxo species requires that a single manganese site be able to accommodate several oxidation states as the water ligand is deprotonated. To study these processes, the preparation and characterization of a new monomeric MnIV–OH complex is described. The MnIV–OH complex completes a series of well characterized Mn–OH and Mn–oxo complexes containing the same primary and secondary coordination spheres; this work thus demonstrates that a single ligand can support mononuclear Mn complexes spanning four different oxidation states (II through V) with oxo and hydroxo ligands that are derived from water. Moreover, we have completed a thermodynamic analysis based on this series of manganese complexes to predict the formation of high valent Mn–oxo species; we demonstrated that the conversion of a MnIV–OH species to a MnV–oxo complex would likely occur via a stepwise proton transfer-electron transfer mechanism. The large dissociation energy for the MnIVO–H bond (∼95 kcal mol−1) diminished the likelihood that other pathways are operative within a biological context. Furthermore, these studies showed that reactions between Mn–OH and Mn–oxo complexes lead to non-productive, one-electron processes suggesting that initial O–O bond formation with the OEC does not involve an Mn–OH unit.

 Please wait while we load your content...
Please wait while we load your content...