Identification of a spin-coupled Mo(iii) in the nitrogenase iron–molybdenum cofactor†
Abstract
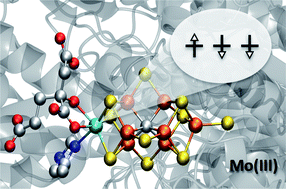
Nitrogenase is a complex enzyme that catalyzes the formation of ammonia utilizing a MoFe7S9C cluster. The presence of a central carbon atom was recently revealed, finally completing the atomic level description of the active site. However, important prerequisites for understanding the mechanism – the total charge, metal oxidation states and electronic structure are unknown. Herein we present high-energy resolution fluorescence detected Mo K-edge X-ray absorption spectroscopy of nitrogenase. Comparison to FeMo model complexes of known oxidation state indicates that the Mo in the FeMo cofactor of nitrogenase is best described as Mo(III), in contrast to the universally accepted Mo(IV) assignment. The oxidation state assignment is supported by theoretical calculations, which reveal the presence of an unusual spin-coupled Mo(III) site. Although so far Mo(III) was not reported to occur in biology the suggestion raises interesting parallels with the known homogenous Mo catalysts for N2 reduction, where a Mo(III) compound is the N2-binding species. It also requires a reassignment of the Fe oxidation states in the cofactor.

 Please wait while we load your content...
Please wait while we load your content...