NMR characterization of cooperativity: fast ligand binding coupled to slow protein dimerization†
Abstract
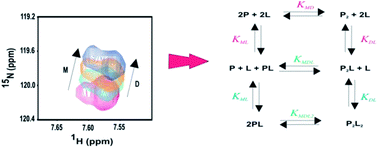
We describe a general approach for analysis of 2D NMR spectra to evaluate the cooperativity of ligand binding and protein dimerization in coupled systems. The approach is applicable to systems in which NMR spectra display separate resonances for monomeric and dimeric species but each resonance shifts in response to ligand binding. Three experimental parameters (monomer chemical shift, dimer chemical shift and relative monomer–dimer peak intensity) are fitted globally, as a function of ligand concentration, to yield equilibrium constants for dimerization, monomer–ligand binding and dimer–ligand binding as well as the cooperativity between ligand binding and dimerization. We have applied the approach to characterise a system in which dimerization of the chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) is coupled to binding of peptides derived from the chemokine receptor CCR2. The global fitting approach allowed evaluation of cooperativity with higher precision than is possible by alternative methods.

 Please wait while we load your content...
Please wait while we load your content...