Functionalised staple linkages for modulating the cellular activity of stapled peptides†
Abstract
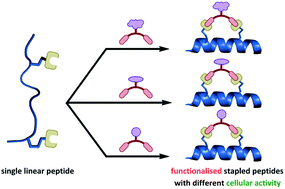
Stapled peptides are a promising class of alpha-helix mimetic inhibitors for protein–protein interactions. We report the divergent synthesis of “functionalised” stapled peptides via an efficient two-component strategy. Starting from a single unprotected diazido peptide, dialkynyl staple linkers bearing different unprotected functional motifs are introduced to create different alpha-helical peptides in one step, functionalised on the staple linkage itself. Applying this concept to the p53/MDM2 interaction, we improve the cell permeability and p53 activating capability of an otherwise impermeable p53 stapled peptide by introducing cationic groups on the staple linkage, rather than modifying the peptide sequence.
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...