Direct monitoring of protein–protein inhibition using nano electrospray ionization mass spectrometry†
Abstract
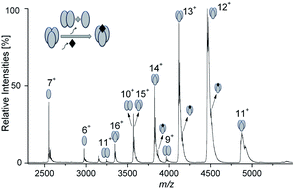
Dissociation of the TNF-alpha trimer caused by the small-molecule inhibitor SPD304 was monitored using native ESI-MS and ion mobility spectrometry (IMS). Upon addition of the inhibitor, our data clearly indicate partial dissociation of the protein into dimers and monomers. The IMS-MS analysis shows that dimeric ions have their own characteristic drift time distributions, which are different from those of the dimer ions originating in the gas phase due to collision-induced dissociation. We show that only one equivalent of the inhibitor binds to the trimeric form. We also investigated inhibition of heterodimer formation of the survival protein Bcl-xL with the cell death-promoting regions of the proteins Bak and Bad, using the small inhibitors ABT737 and ABT263. We found that ABT737 is more potent than ABT263 in preventing the heterodimerization between Bcl-xL and the Bak and Bad derived BH3 peptides. We could also monitor the mode of binding, which in this case is competitive. These results indicate that native ESI-MS can be widely used to study the inhibition of other relevant protein–protein interactions (PPIs), and provide a good basis for further improvement and identification of small-molecule PPI inhibitors.

 Please wait while we load your content...
Please wait while we load your content...