Two-electron versus one-electron reduction of chalcogens by uranium(iii): synthesis of a terminal U(v) persulfide complex†
Abstract
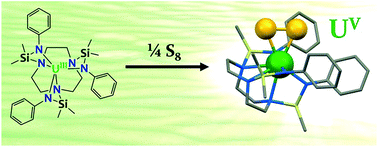
The reaction of the tripodal tris-amido U(III) complex [U{(SiMe2NPh)3–tacn}] (tacn = 1,4,7-triazacyclononane), 1, with 0.0625 and 0.25 equiv. of elemental sulfur affords the sulfide-bridged U(IV) complex [{U((SiMe2NPh)3–tacn)}2(μ-S)], 2, and the terminal persulfide U(V) complex [U{(SiMe2NPh)3–tacn}(η2-S2)], 4, respectively, in good yield. Two different electronic structures, U(V) persulfide and U(IV) supersulfide, were computed for complex 4 at the DFT level. The results show that complex 4 is best described as a U(V) persulfide species with a significant sulfur contribution. X-ray, magnetism and electrochemistry data support this description. Complex 4 is the first example of a terminal U(V) persulfide and of a two-electron reduction of S8 by a U(III) complex. Complex 4 behaves as a S-atom transfer agent when reacted with PPh3, affording the persulfide-bridged diuranium(IV) complex [{U((SiMe2NPh)3–tacn)}2(μ-η2:η2-S2)], 5, and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3.
PPh3.

 Please wait while we load your content...
Please wait while we load your content...