Discovery of a family of γ-aminobutyrate ureas via rational derepression of a silent bacterial gene cluster†
Abstract
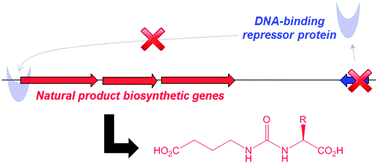
Gaburedins, a family of γ-aminobutyrate (GABA)-derived ureas, have been discovered by deletion of gbnR, an arpA-like putative transcriptional repressor in Streptomyces venezuelae ATCC 10712. Comparison of metabolite profiles in the wild type and mutant strains revealed six metabolites in the mutant that are lacking from the wild type. The structure of gaburedin A was established by HRMS combined with 1- and 2-D NMR spectroscopy and was confirmed by total synthesis. The other metabolites were confirmed as congeners using HRMS, MS/MS and feeding of putative biosynthetic precursors. Two genes, gbnA and gbnB, are proposed to be involved in gaburedin biosynthesis. Consistent with this hypothesis, deletion of gbnB in the gbnR mutant abolished gaburedin production. This is the first report to disclose the discovery of novel natural products via rational deletion of a putative pathway-specific regulatory gene.

 Please wait while we load your content...
Please wait while we load your content...