Insight into bicyclic thiopeptide biosynthesis benefited from development of a uniform approach for molecular engineering and production improvement†
Abstract
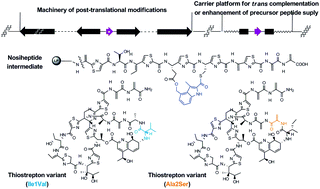
The ribosomal origin of thiopeptide antibiotics, a class of sulfur-rich and highly modified poly(thi)azolyl natural products, has recently been uncovered and features complex post-translational modifications (PTMs) of a precursor peptide. Based on molecular engineering and production improvement, we report insight into the biosynthesis of two bicyclic thiopeptide compounds, thiostrepton and nosiheptide. The PTMs of thiostrepton tolerate variations in the first two amino acids of the core peptide part of the precursor peptide: (1) the mutation of Ile1 to Val had no apparent effect on molecular maturation, suggesting that attachment of the quinaldic moiety at position 1 is not residue-dependent for the construction of the side ring system; and (2) the change of Ala2 to Ser led exclusively to the production of an analog that bears a corresponding dehydroamino acid residue, indicating that dehydration at position 2 is site-selective or that the oxazoline formed by cyclodehydration is inaccessible for maturation. For nosiheptide biosynthesis in particular, we provide the first structural evidence that construction of the specific side ring system precedes formation of the common central heterocycle domain and therefore propose that formation of a characteristic thiopeptide framework interweaves both common and specific PTMs that are interdependent. The above efforts benefited from the development of a uniform approach to examine the effectiveness for trans expression of gene encoding precursor peptides and associated PTM capacity. This approach is independent of knowledge regarding organism-specific regulatory mechanisms and potentially applicable to other systems that produce ribosomally synthesized peptide natural products.

 Please wait while we load your content...
Please wait while we load your content...