Computational insight of the mechanism of Algar–Flynn–Oyamada (AFO) reaction†
Abstract
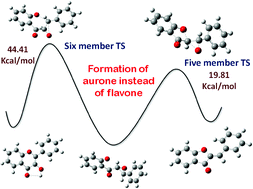
The present DFT investigation supports a previous conclusion of Dean et al. that hydroxylation occurs without epoxide intermediate at room temperature due to a strong electrostatic interaction of peroxide ions with π electrons of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of chalcone, and 3-hydroxyflavone has been found to be the major product. The calculated activation energy difference (ΔG#) of initial enolization followed by hydroxylation or simultaneous cyclization and hydroxylation has been found to be negligible (∼4 kcal mol−1). On the other hand, epoxide formation requires significant activation energy, which is supposed to occur at high temperatures. In addition, if epoxide is formed, the ring opens by an attack of phenolic oxygen, occurring preferentially at α position via a five-member transition state due to a low activation barrier height (19.82 kcal mol−1 in the gas phase and 19.55 kcal mol−1 in ethanol) compared to that of a six-member transition state (44.41 kcal mol−1 at B3LYP in the gas phase and 38.55 kcal mol−1 in ethanol). It is also observed that the solvation study does not affect the main conclusion of the paper. These findings also support the previous observation of Dean et al. Predicted ΔG# in different DFT functionals are consistent, although the total energy is significantly different.
C bonds of chalcone, and 3-hydroxyflavone has been found to be the major product. The calculated activation energy difference (ΔG#) of initial enolization followed by hydroxylation or simultaneous cyclization and hydroxylation has been found to be negligible (∼4 kcal mol−1). On the other hand, epoxide formation requires significant activation energy, which is supposed to occur at high temperatures. In addition, if epoxide is formed, the ring opens by an attack of phenolic oxygen, occurring preferentially at α position via a five-member transition state due to a low activation barrier height (19.82 kcal mol−1 in the gas phase and 19.55 kcal mol−1 in ethanol) compared to that of a six-member transition state (44.41 kcal mol−1 at B3LYP in the gas phase and 38.55 kcal mol−1 in ethanol). It is also observed that the solvation study does not affect the main conclusion of the paper. These findings also support the previous observation of Dean et al. Predicted ΔG# in different DFT functionals are consistent, although the total energy is significantly different.

 Please wait while we load your content...
Please wait while we load your content...