Endo-benzonorbornen-2-ol as an efficient non-natural chiral auxiliary in the asymmetric aza-Diels–Alder reactions between cyclopentadiene and (1-phenylethyl)iminoacetates†
Abstract
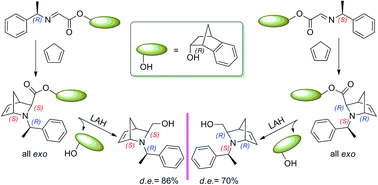
Exo-2-azabicyclo[2.2.1]hept-5-enes were obtained by cycloaddition reactions between cyclopentadiene and protonated (S)- and (R)-1-phenylethylimines of (1R,endo)-benzonorbornen-2-yl glyoxylate. The non-natural (1R,endo)-benzonorbornen-2-ol proved to be an efficient chiral auxiliary in these asymmetric aza-Diels–Alder reactions, which afforded only exo-cycloadducts with 1S : 1R diastereomeric ratios of 15 : 85 for (S)-(1-phenylethyl)imine and 93 : 7 for (R)-(1-phenylethyl)imine. The obtained diastereoisomers were effortlessly and efficiently isolated using a chromatographic column. The absolute configuration of all the formed adducts was unequivocally assigned through NMR, specific optical rotation and X-ray data.

 Please wait while we load your content...
Please wait while we load your content...