Understanding the selectivity in the formation of δ-lactams vs. β-lactams in the Staudinger reactions of chloro-cyan-ketene with unsaturated imines. A DFT study†
Abstract
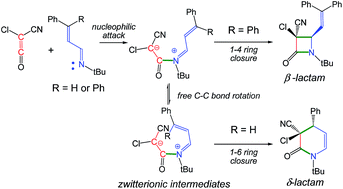
The reactions of chloro-cyan-ketene with two phenyl substituted unsaturated imines yielding β- or δ-lactams have been investigated using DFT methods at the MPWB1K/6-311G(d,p) level in diethyl ether. The reactions are initialised by the nucleophilic attack of the unsaturated imines on the ketene with formation of zwitterionic intermediates. The subsequent C–C single bond formation at the imine carbon or at the β-conjugated position enables the formation of β- or δ-lactams. Analysis of the energies involved in the two competitive channels explains the selectivity experimentally observed; in the absence of any steric hindrance, formation of δ-lactams is favoured over the formation of β-lactams. ELF topological analysis allows explaining the bonding changes along the two competitive channels. While formation of the N–C bond takes place by participation of the nitrogen lone pair, formation of the C–C bonds takes place through a retrodonation process involving the C–C double bond of the ketene and the C–N or C–C double bonds of the unsaturated imine. ELF topological analysis makes it possible to rule out an electrocyclic mechanism for the cyclisation step.

 Please wait while we load your content...
Please wait while we load your content...