Self-assembled monolayers of terminal acetylenes as replacements for thiols in bottom-up tunneling junctions†
Abstract
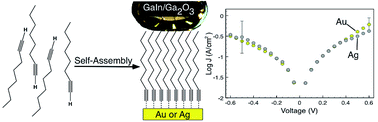
Why use thiols in Molecular Electronics? They stink, oxidize readily, poison catalysts, and often require nontrivial protection/deprotection chemistry. In this communication we demonstrate the fabrication of tunneling junctions formed by contact of self-assembled monolayers (SAMs) of terminal alkynes onto silver and gold substrates. The SAMs form spontaneously upon exposure of the substrates to ethanolic solutions of the alkynes. Characterization by vibrational spectroscopy, XPS, and contact angles shows that the packing of the SAMs is nearly identical to those formed from equivalent thiols. Electrical characterization of the junctions revealed virtually no differences between SAMs on gold and silver, yielding βAu = 1.17 ± 0.04nC−1, J0 = (2.836 ± 0.001) × 103 A cm−2 for Au, and βAg = 1.23 ± 0.09nC−1, J0 = (4.722 ± 0.002) × 103 A cm−2 for Ag. These values are in excellent agreement with junctions formed from alkanethiols of the same lengths as the alkynes, suggesting that there is no functional difference between thiols and alkynes as anchoring groups for SAMs. Yet alkynes are synthetically versatile, do not poison catalysts, are not odorous, and do not spontaneously oxidize, which are all attractive features for use in Molecular Electronics.

 Please wait while we load your content...
Please wait while we load your content...