A mononuclear copper electrocatalyst for both water reduction and oxidation†
Abstract
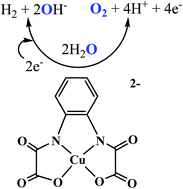
The oxidation and reduction of water is a key challenge in the production of chemical fuels from electricity. Although several catalysts have been developed for these reactions, substantial challenges remain towards the ultimate goal of an efficient, inexpensive and robust electrocatalyst. There is as yet no report on both water oxidation and reduction by the same catalyst. Reported here is a soluble copper-based catalyst, Na2[Cu(opba)] 1 (opba: o-phenylenebis(oxamato)) for water oxidation and reduction. Water oxidation occurs at an overpotential of 636 mV vs. SHE to give O2 with a turnover frequency (TOF) of ∼1.13 s−1. Electrochemical studies also indicate that 1 is a soluble molecular species, and that this is the most rapid homogeneous water-reduction catalyst, with a TOF of 1331.7 (pH 7.0) moles of hydrogen per mole of catalyst per hour in a pH 7.0 buffer at an overpotential of 788 mV vs. SHE. Sustained water reduction catalysis occurs at glassy carbon (GC) to give H2 over a 36 h electrolysis period with 96.5% Faradaic yield and no observable decomposition of the catalyst.
- This article is part of the themed collection: Luminescence and photophysical properties of metal complexes

 Please wait while we load your content...
Please wait while we load your content...