Facile synthesis of nickel hydroxide–graphene nanocomposites for insulin detection with enhanced electro-oxidation properties
Abstract
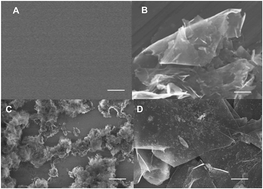
This study describes a facile and effective one-pot route to synthesize structurally uniform and electrochemically active nickel hydroxide–graphene nanocomposites (Ni(OH)2–GN) and investigates the electrocatalytic activity toward the oxidation of insulin. Graphene here was used to tether Ni2+ precursor onto surfaces and eventually grow Ni(OH)2 nanoparticles to form hybrid materials. The synthetic Ni(OH)2–GN nanocomposite has a uniform surface distribution, which was characterized with scanning electron microscopy (SEM). Moreover, the composition of synthetic Ni(OH)2–GN nanocomposite was characterized by X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FT-IR spectra). The Ni(OH)2–GN were electrochemically treated in 0.1 M NaOH solution through cyclic voltammograms, and then gradually transited into nickel oxyhydroxide–graphene nanocomposites (NiOOH–GN), which demonstrated high catalytic activity and improved stability to insulin oxidation. The steady-state current response increases linearly with insulin concentration from 800 nM to 6400 nM with a fast response time of less than 2 s and a detection limit of 200 nM. The excellent performance of insulin sensor, including long term stability, can be ascribed to the synergistic effects of the large surface area (resulting in high loading ability), dispersing ability and conductivity of graphene and the large surface-to-volume ratio and electrocatalytic activity of Ni(OH)2 nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...